ONCE Dialyze®
Medical food for dialysis patients (Vanilla flavour).
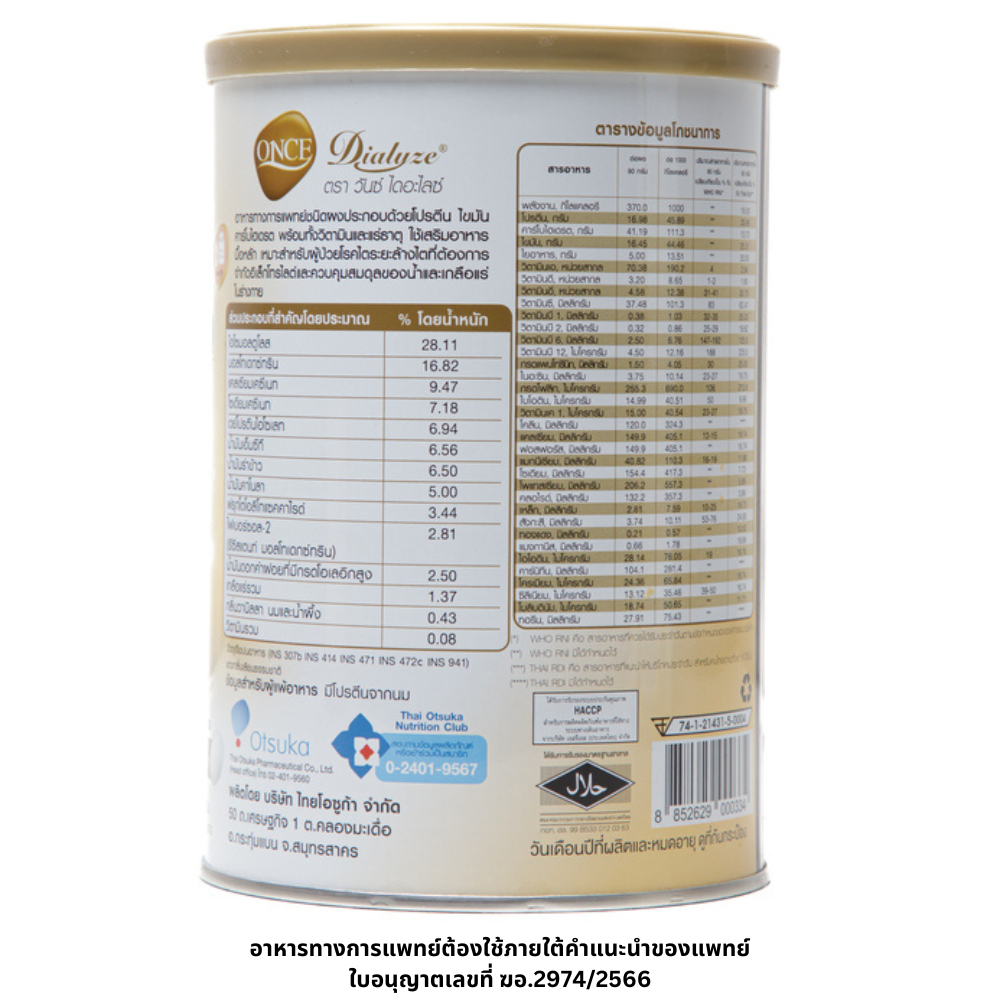
Once Dialyze® powder consists of proteins, fats, carbohydrates, vitamins and minerals for supplement the meal. Optimum for dialysis patients who need to control electrolyte limit, water and mineral balance.
• High proteins which suitable for dialysis patients.
• Good quality of proteins from whey protein isolate and casein.
• Vitamin A, sodium, potassium and phosporus that optimum for dialysis patients.
• High energy
• Contains Isomaltulose, fibersol-2 and fructooligosaccharide.
• No sucrose added, Lactose free.
Description
• Caloric distribution from 18% proteins, 42% carbohydrates, 40% fats.
• Supplement for daily meal.
• Consists of whey protein isolate and casein; contributes to a source of essential amino acids for body proteins synthesis
• Contains canola oil, safflower oil with high oleic acid, rice bran oil and MCT oil as source of energy.
• Contains dietary fiber; contributes to an increase in fecal bulk and stimulates the bowel movement.
Net weight 400 grams.
-Food serial no.74-1-21431-5-0004
-Manufacturer is certified HACCP for enteral nutrition products by SGS (Thailand) Limited.
-Registration no.CICOT.HL.99 B533 012 03 63
-Manufactured by:
Thai Otsuka Pharmaceutical Co., Ltd.
50, Sethakij 1 Road, Khlong Madua, Kra Tum Ban, Samutsakorn, Thailand.
Food advertising no.3001/2566
 Thai Otsuka Online Shop
Thai Otsuka Online Shop